Materials for Advanced Lithium Based Batteries (LIB, LIS, LIM)...
This section starts based on our native 'Ionic Systems' experience and deals with electrolytes / electrolyte salts / additives for Li-Ion-, Li-Sulfur- and Li-Metal-Batteries.
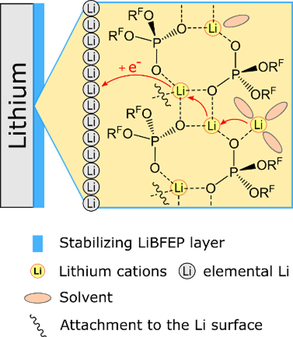
Adaptive Self-healing Artificial SEI #I: In our opening work
“An Artificial SEI Layer Based on an Inorganic Coordination Polymer with Self-Healing Ability for Long-Lived Rechargeable Lithium Metal Batteries.” in Batt. & Supercaps 2022, e202100347. https://doi.org/10.1002/batt.202100347
we report that the deposition of a fluoro-organic phosphoric acid on Li metal results in the formation of an artificial SEI layer on its sur-face. This layer enables a substantial cycle lifetime enhancement in Li-Li symmetric cells. The main constituent of the layer is a Li-ion conducting coordination polymer with adaptive self-healing ability.
LiBFEP = Li[O2P(OCH2CF3)2]

Adaptive Self-healing Artificial SEI #II:
In the accompanying Full Paper currently under revision,
"Towards Realistic Full Cells with Protected Lithium-Metal-Anodes: The Effect of an Adaptive Self-healing Artificial SEI." By S. Burger, J. Skrotzki, J. Büttner, W. Beichel, P. Klose, A. Welle, A. Fischer, and I. Krossing*. In Revision.
the successful formation of a LiBFEP-based artificial solid electrolyte interphase (SEI) on thin Li-metal anodes is confirmed using various characterization methods. Extended electrochemical cycling tests in full cells show a positive effect of the artificial SEI and result in tripling of the cell’s lifetime. Scanning electron microscopy measurements reveal the structural stabilization of the artificial SEI during and after the cycling tests.
Low Ion-Pairing Conducting Salt for LIS: The detrimental polysulfide shuttle associated with Li-S batteries is alleviated (without the addition of LiNO3) through the combined use of a non-polar sulfonamide solvent and low-ion pairing salt as the electrolyte. This combination transforms to a sparingly solvating electrolyte at 50 °C and sustains close to theoretical capacity at rates of C/5 with a coulombic efficiency of 99.7 %.
Novel WCA-based Conducting Salts…? The difluorophosphato ligand (O2PF2) was used for the synthesis of borates and aluminates of the [M(O2PF2)x]y–-type in order to generate new weakly coordinating anions. The preparation and characterization of the [B(O2PF2)4]– anion with various different counterions as well as Li3[Al(O2PF2)6] and Al(O2PF2)3 is described.
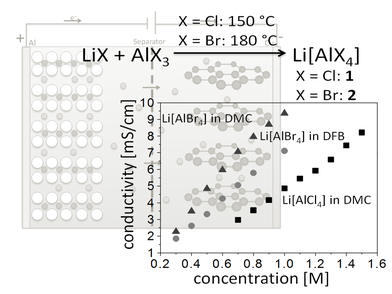
Li[AlX4] (X = Cl, Br) as Conducting Salts: The lithium salts
of the bromo- and chloro-aluminates were tested and fully characterized for application as electrolytes for Lithium-ion and Lithium-sulfur batteries in conventionally used solvents as well as
o-difluorobenzene as possible alternative. Li[AlBr4]
had very high conductivties in this latter solvent. The lattice enthalpies of these salts were also investigated.
Eur. J. Inorg. Chem. 2015, 19, 3128–3138.
Conducting Salt for Li-Batteries…? Li[B(OTfe)4] (OTfe = OCH2CF3) was synthesized as a water and thermally stable lithium ion electrolyte salt. The electrochemical stability in EC/DMC and in DME lies outside the limits of the solvents. As electrolyte salt, it exhibits good conductivities in many solutions. It was tested in LiSB-Batteries and showed good performance.
ChemPhysChem 2015, 16, 666–675.
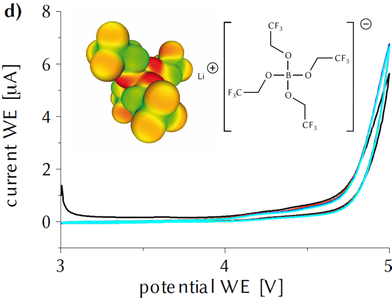

This compound now works as a novel high voltage additive for LNMO/graphite cells.
J. Electrochem. Soc. 2018, 165, A2569-A2576.
Coordination-Polymer based Gel-Electrolytes: The lithium bis(fluoroalkyl)phosphates (1) and (2) were used to prepare novel coordination-polymer based gel-electrolytes that hold conductivity records (see picture). A first performance testing of a cell with addition of 0.25 mol L–1 of (2) proved them to be stable for the application in Lithium-ion batteries (NCM 111 cathode) with some hints that they may also induce formation of a high voltage SEI layer.
ChemElectroChem 2016, 3, 774–782.