Experimental Realization - The 'ideal' ILSB...

Using the setup of an 'ideal' ionic liquid salt bridge (ILSB), we showed that one can measure ideal Nernst’ian behavior between many solvents.
For the construction of the PPM, one needs Gibbs energies of solvation of single ions ΔsolvG°(i, S). Because of their electric charge, their determination is anything but straightforward. Also the related Gibbs energies of transfer ΔtrG°(i, S1→S2) = ΔsolvG°(i, S2) − ΔsolvG°(i, S1) could hitherto only be measured using so-called extra-thermodynamic assumptions. Most notably the TATB-assumption.
We therefore derived a concept to measure these quantities without extrathermodynamic assumption. This essentially justifies single ion thermodynamics, a more than 100 year old problem.
The main idea is to use an “ideal” ionic liquid for the salt bridge. I.e., an IL that has identical diffusion constants and an ionicity of at best 100 %.
The next table shows the by PGSTE-NMR measured diffusion constants of the selected 'ideal' IL as contrasted by a classical imidazolium IL.
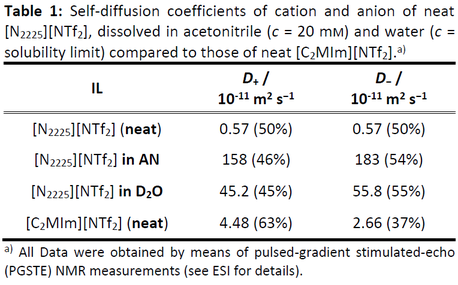
Realization: Angew. Chem., Int. Ed. 2018, 57, 2348–2352.
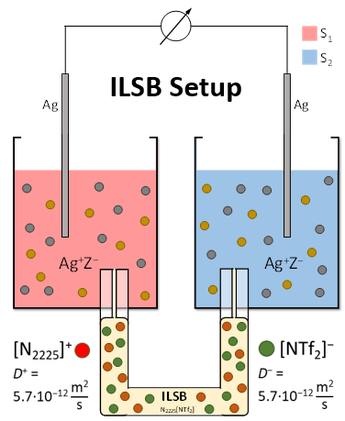
This 'ideal' ILSB-methodology turned out very successful and allows to directly measure the desired transfer magnitudes in straight forward electrochemical measurements that are in addition suitable to be checked for consistency using the statistical Network Analysis approach described in the section 'Network Analysis'.
